Nov 18, 2015
Blog Advanced Materials New Material Inspired by Nature Could Turn Water into Fuel
Scientists have theoretically designed a new material that could help supply the world with clean energy by turning water into fuel using the power of the sun.
Chemists at the U.K.-based University of Reading say a new catalyst, which mimics the way plants absorb energy from the sun, could make the energy-sapping job of splitting water into hydrogen and oxygen fairly easy. The findings, published in the Journal of Materials Chemistry, suggest the complex material potentially could be used not only to produce hydrogen for fuel cells, but also to turn carbon dioxide from the air into a carbon-based fuel like methanol.
“Finding a material that can help create readily available fuels is one of the holy grails of science," said Ricardo Grau-Crespo, Ph.D., who led the team that made the discovery. “While we still have a long way to go, our new findings could be a significant step forward in the search for cheaper, environmentally-friendly fuels to power the future."
ARTIFICIAL PHOTOSYNTHESIS INSPIRED… NATURALLY
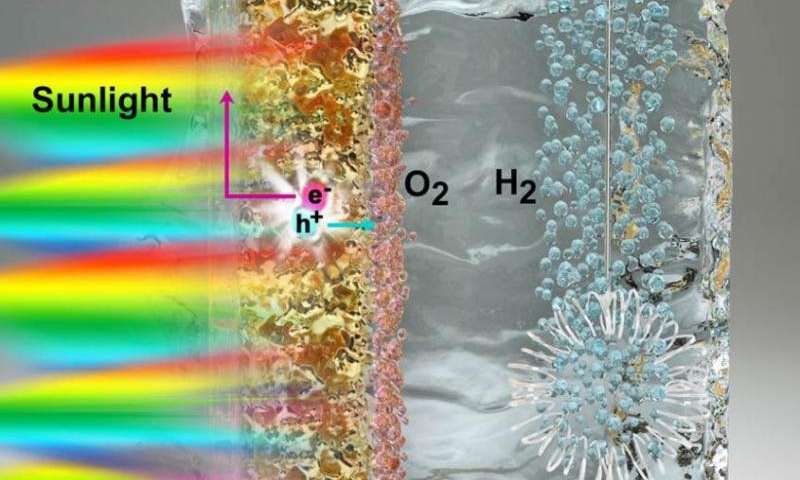
Splitting water into hydrogen and oxygen is an energy-intensive process that draws more energy from electricity than what is produced in usable fuel.
To make the process more efficient, scientists use a photocatalyst—a material that absorbs light from the sun and uses it to excite electrons to higher energy levels. These excited electrons, and the empty spaces they leave behind, are capable of driving forward the two half-reactions required to split water into oxygen and hydrogen.
But finding a good photocatalyst poses a challenge because it must have the precise electronic structure to catalyze these reactions. Titanium oxide, one of the best photocatalyst materials available, is too inefficient to produce more than a tiny amount of hydrogen because it can only absorb energy from ultraviolet light.
When designing the study, Grau-Crespo said his team relied on nature for guidance, specifically how plants use chlorophyll to convert sunlight into chemical energy.
"Our research is inspired by nature, as porphyrin is related to chlorophylls, the green pigments which allow plants to convert sunlight into chemical energy,” he said.
The team used supercomputer simulations to identify optimal candidates for fuel production reaction. They concluded that some metal-organic frameworks, which combine metal atoms and organic molecules, exhibited the ideal structure necessary to catalyze these reactions.
"The challenge now is to incorporate these wonderful natural catalysts into materials capable of doing the specific chemical job we need. If we can do this, it could lead to highly-efficient conversion of solar energy to chemical energy - providing a clean, storable and transferrable source of energy."
GLOBAL ENERGY DEMAND SPURRING RESEARCH FOR ALTERNATIVE POWER SOURCES
Margareth Gagliardi, chief research analyst in Advanced Materials for BCC Research, said research studies like the one conducted by Grau-Crespo and his team could have enormous impact both on the environment and world’s energy needs.
The traditional sources of energy based on nonrenewable fossil fuels such as coal, gas and petroleum generate high levels of CO2 and particulate pollution, according to Gagliardi. As emerging countries continue their steady transition into advanced consumer societies, their energy needs also increase, increasing demand for the development of alternative fuel sources, including artificial photosynthesis, she said.
“Energy consumption is forecast to expand by more than 50% during the next 35 years,” Gagliardi explained. “In fact, (artificial photosynthesis) would be able to reduce potentially dangerous levels of carbon dioxide in the atmosphere and, at the same time, produce cleaner energy source alternatives to nonrenewable fossil fuels such as coal and oil, helping to satisfy the world’s increasing demand for energy.”
The global market for photocatalyst-based products is projected to reach nearly $1.6 billion and $2.9 billion in 2015 and 2020, respectively, reflecting a five-year compound annual growth rate (CAGR) of 12.6%, according to BCC Research.

Oxygen scavengers play a crucial role in preventing oxidative reactions that can...

The textile industry is a major contributor to carbon footprint and waste genera...

In recent years, the 3D printing industry has witnessed a remarkable surge in in...

We are your trusted research partner, providing actionable insights and custom consulting across life sciences, advanced materials, and technology. Allow BCC Research to nurture your smartest business decisions today, tomorrow, and beyond.
Contact UsBCC Research provides objective, unbiased measurement and assessment of market opportunities with detailed market research reports. Our experienced industry analysts assess growth opportunities, market sizing, technologies, applications, supply chains and companies with the singular goal of helping you make informed business decisions, free of noise and hype.