Sep 22, 2015
Blog Life Sciences Researchers Identify Promising Therapy for Rare Immune Disorder
A multi-institutional team of researchers have identified a promising therapy for a life-threatening genetic immune disorder that lacks effective treatments.
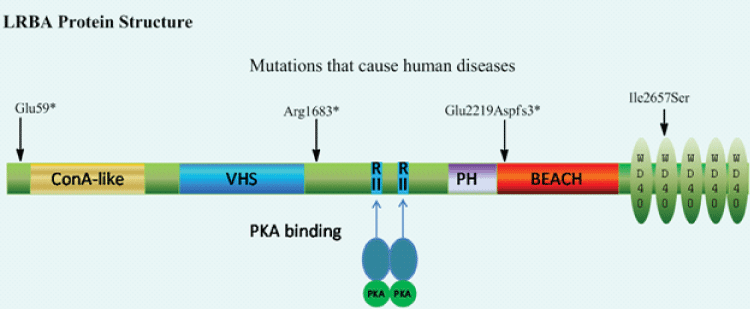
Led by Cincinnati Children's Hospital Medical Center and the National Institute of Allergy and Infectious Diseases (NIAID), researchers found that abatacept, a drug already approved for treating rheumatoid arthritis, may offer effective long-term therapy for LRBA deficiency, a rare immune disorder caused by mutations in the LRBA gene.
LRBA deficiency can be associated with autoimmune diseases, chronic infections and interstitial lung disease, which complicates breathing. In 2012, researchers first identified disease-causing mutations in LRBA, offering a specific diagnosis for patients previously diagnosed with common variable immune deficiency (CVID).
The discovery prompted researchers at NIAID, Cincinnati Children's and their collaborating institutions to explore LRBA's biological role in helping maintain healthy immune function. Working with clues from abatacept therapy, which showed promise for CVID patients before their diagnosis with LRBA deficiency, the authors focused on a protein in immune cells called CTLA4, an immune cell brake.
LRBA deficiency results in a loss of the CTLA4 protein. Abatacept treatment works by mimicking the function of CTLA4 in regulatory T cells, thus maintaining sufficient levels of CTLA4 by preventing its degradation.
In the study, six of nine patients with LRBA deficiency received treatment with abatacept for a period ranging from six to eight months. One patient received hydoxychlorquine without abatacept. Seven treated patients experienced improved lung function and reduced autoimmunity, among other benefits. Not all symptoms improved in the patients, according to the researchers.
"There have been no effective treatments for LRBA deficiency, but this study identifies a therapy that appears to be highly effective at reversing life-threatening infiltrative and autoimmune disease," said Michael Jordan, MD, co-senior investigator on the study. "The study findings provide a clear rationale for a prospective clinical trial to further test what may be an effective long-term treatment for this disorder."
The authors report their data also suggest that hydroxychloroquin may work as an inexpensive alternative in patients with LRBA deficiency or other autoimmune disorders linked to reduced levels of CTLA
The global orphan drug market, which totaled $117.3 billion in 2013, is projected to reach $123 billion and $191 billion in 2014 and 2019, respectively, reflecting a compound annual growth rate (CAGR) of 9.2% from 2014-2019.
Related BCC Research Report:
Global Markets for Orphan Drugs (PHM038E)
In today’s fast-paced biomedical world, researchers and pharmaceutical companies...
Radiopharmaceuticals represent a cutting-edge frontier in modern medicine, offer...
Implantable Remote Patient Monitoring (IRPM) devices are revolutionizing healthc...

We are your trusted research partner, providing actionable insights and custom consulting across life sciences, advanced materials, and technology. Allow BCC Research to nurture your smartest business decisions today, tomorrow, and beyond.
Contact UsBCC Research provides objective, unbiased measurement and assessment of market opportunities with detailed market research reports. Our experienced industry analysts assess growth opportunities, market sizing, technologies, applications, supply chains and companies with the singular goal of helping you make informed business decisions, free of noise and hype.