Sep 6, 2024
Blog Life Sciences In Vitro Diagnostics Quality Controls: Safeguarding Precision in Diagnostics Worldwide
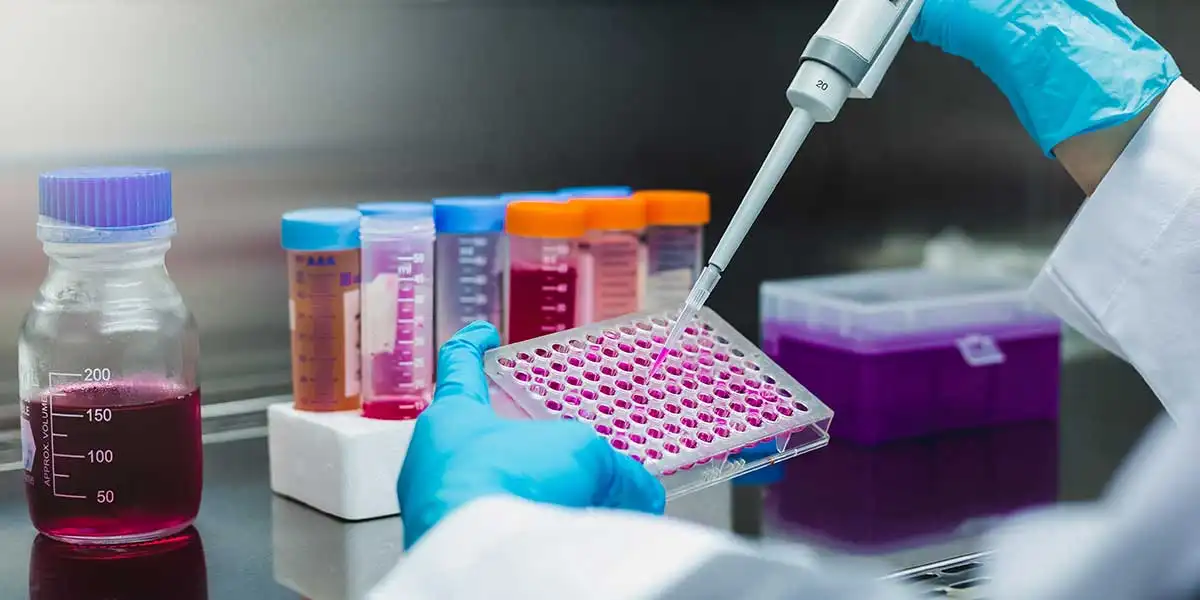
In vitro diagnostics (IVD) have become indispensable in modern healthcare, helping clinicians diagnose, monitor, and treat diseases precisely. These tests are crucial in patient care, from detecting infectious diseases to assessing chronic conditions. But behind every accurate and reliable test lies a robust system of quality controls (QCs), ensuring that these diagnostic tools deliver consistent and trustworthy results.
As the global In vitro diagnostics market expands, quality controls are becoming more critical than ever.

According to BCC Research, the global market for in vitro diagnostics quality controls is expected to grow from $1.5 billion in 2024 to $2.1 billion by the end of 2029 at a compound annual growth rate (CAGR) of 7.8% during the forecast period of 2024 to 2029.
In vitro diagnostics quality controls are reference materials used to validate the accuracy and precision of diagnostic tests. They simulate patient samples and are tested alongside specimens to ensure the diagnostic process functions correctly. These controls can detect deviations or errors in the test results, allowing laboratories to make necessary adjustments before analyzing patient samples.
With adequate quality controls, the reliability of diagnostic tests could be protected, leading to misdiagnosis, incorrect treatment plans, and potentially harmful outcomes for patients.
The global market for in vitro diagnostics quality controls is expected to grow from $1.5 billion in 2024 and is projected to reach $2.1 billion by the end of 2029, at a compound annual growth rate (CAGR) of 7.8% during the forecast period of 2024 to 2029.
The global In vitro diagnostics market is growing rapidly, driven by technological advances, an aging population, and the increasing prevalence of chronic and infectious diseases. As new diagnostic tests are developed, the need for rigorous quality controls has never been greater. Here's why:
Regulatory Requirements: International regulatory agencies with strict guidelines for In vitro diagnostics quality standards include the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) in the United States. Before being put on the market, manufacturers must prove that their testing meets these requirements. As a result, there is an increasing need for superior controls that can guarantee adherence to these rules.
Technological Advancements: The test's complexity increases in tandem with the advancement of IVD technology. Modern platforms, assays, and techniques call for sophisticated quality controls. For instance, exact and sensitive controls are required to confirm the accuracy of molecular diagnostics, which identify genetic material.
Global Expansion: The In vitro diagnostics market is expanding outside wealthy nations. Diagnostic testing is expanding quickly in emerging economies in Asia, Latin America, and Africa. Due to this expansion, the need for consistent quality controls that can be applied in various testing conditions and geographical areas has increased.
The In vitro diagnostics quality controls market is poised for significant growth, driven by several key trends:
Personalized Medicine: Accurate and trustworthy diagnostics are critical as healthcare transitions to personalized medicine, in which patients' regimens are customized according to their genetic composition. There is a great need for quality controls that can guarantee the precision of these specialist tests.
Point-of-Care Testing: The emergence of point-of-care (POC) testing, which involves performing diagnostic tests at or close to the patient care site, is revolutionizing healthcare delivery. Portable and user-friendly quality controls are necessary to guarantee that POC tests yield correct findings in various locations, including remote clinics and hospitals.
Automation and AI Integration: The integration of automation and artificial intelligence (AI) in diagnostic laboratories is on the rise. Advanced quality controls compatible with AI-driven diagnostics and automated systems are becoming increasingly necessary due to this change.
Sustainability: The effects of healthcare procedures, such as diagnostic testing, on the environment are becoming more well-recognized. Manufacturers are investigating eco-friendly quality standards that decrease waste and the environmental impact of diagnostic testing.
While the future looks promising, the In vitro diagnostics quality controls market also faces challenges:
Cost Pressures: High-quality control development and production might be costly. The expense of these necessary supplies may be prohibitive for laboratories, particularly in environments with limited resources. For producers, finding a balance between cost and quality is still difficult.
Standardization: As the in vitro diagnostics market expands globally, it is imperative to guarantee standardization across various locations. Different regulatory frameworks, testing conditions, and medical procedures can make creating relevant quality controls everywhere more difficult.
Supply Chain Disruptions: The COVID-19 outbreak highlighted weak points in international supply systems. The reliability of diagnostic tests depends on a consistent supply of quality controls, especially during public health emergencies.
The stability and continuous expansion of the in vitro diagnostics industry largely depend on the global In-vitro diagnostics quality control market. Robust quality controls will become increasingly necessary as the need for precise and accurate diagnoses grows. The international expansion of diagnostic testing, regulatory constraints, and technology developments will all contribute to the In vitro diagnostics quality control market's significant influence on the direction of healthcare in the future.
Investing in quality controls is not just a legal necessity for laboratories, healthcare providers, and manufacturers but also a commitment to patient safety and providing high-quality treatment.
Consider becoming a member of the BCC Research library and gain access to our full catalog of market research reports in your industry. Not seeing what you are looking for? We offer custom solutions too, including our new product line: Custom Intelligence Services.
Contact us today to find out more.

Sandeep is a Senior Executive in Marketing Operations at BCC Research, proficiently serving as a graphic designer and content creative specialist. His expertise extends to AutoCAD and Revit, and he has made valuable contributions to the event industry with his design skills.
In today’s fast-paced biomedical world, researchers and pharmaceutical companies...
Radiopharmaceuticals represent a cutting-edge frontier in modern medicine, offer...
Implantable Remote Patient Monitoring (IRPM) devices are revolutionizing healthc...

We are your trusted research partner, providing actionable insights and custom consulting across life sciences, advanced materials, and technology. Allow BCC Research to nurture your smartest business decisions today, tomorrow, and beyond.
Contact UsBCC Research provides objective, unbiased measurement and assessment of market opportunities with detailed market research reports. Our experienced industry analysts assess growth opportunities, market sizing, technologies, applications, supply chains and companies with the singular goal of helping you make informed business decisions, free of noise and hype.