Researchers at the University of Maryland have pioneered a flexible, solid-state, ion-conducting membrane based on a 3D Li-ion conducting ceramic nanofiber network. High capacity, high safety, and long lifespan are three of the most important key factors to developing rechargeable lithium batteries for applications including portable electronics and electrical vehicles.
The membrane shows superior thermal stability and electrochemical stability to high voltage, and can replace conventional flammable organic liquid electrolyte systems in lithium-ion batteries. The research was published in the
Proceeding of the National Academy of Sciences (PNAS).
As noted by Belle Dumé, editor with
NanotechWeb.org, a website of the UK Institute of Physics, researchers are seeking to boost the amount of energy that can be stored in the batteries.
“To this end, they are developing anodes made from metallic lithium anodes rather than the conventional ion intercalation anode materials. Lithium has the highest specific capacity and the lowest negative electrochemical potential, which could maximize the capacity density and operating voltage window for increased battery energy density,” Dumé writes.
WHAT ARE LITHIUM-ION BATTERIES?
Lithium-ion (Li-ion) chemistry represents the pinnacle for batteries of energy density (deliverable energy divided by mass) and power density (deliverable power divided by mass), and as a result, batteries based upon this chemistry dominate the portable electronics market.
Typically, the electrolyte is a volatile organic compound, but solid-state electrolytes and ionic liquids with reduced flammability are under investigation,
according to Christopher Maara, a BCC Research analyst. The exclusion of oxygen and water increases the manufacturing and materials cost of lithium-ion batteries compared to aqueous cells. Lithium chemistry is inherently less safe than aqueous chemistries because of the higher voltage and (at least at present) the flammability of the volatile electrolyte.
“Li-ion batteries offer high energy and power densities, efficiency, and expected life,”
explains Maara. “This energy capacity per weight makes them very convenient for portable devices, electric vehicles and the like. The reliability of Li-ion batteries has been proven in many grid-tied projects around the world. As such, they will play a major role over the next two years because it is one of the only rechargeable battery chemistry’s appropriate for grid-scale and commercial-scale deployment.”
Today’s electric vehicles are commonly powered by rechargeable lithium-ion batteries, which are also being used to store renewable energy. And the research and development
directed toward lithium-ion has made it the fastest-growing grid-scale battery storage electrochemistry.
LITHIUM-ION BATTERY COMPOSITION
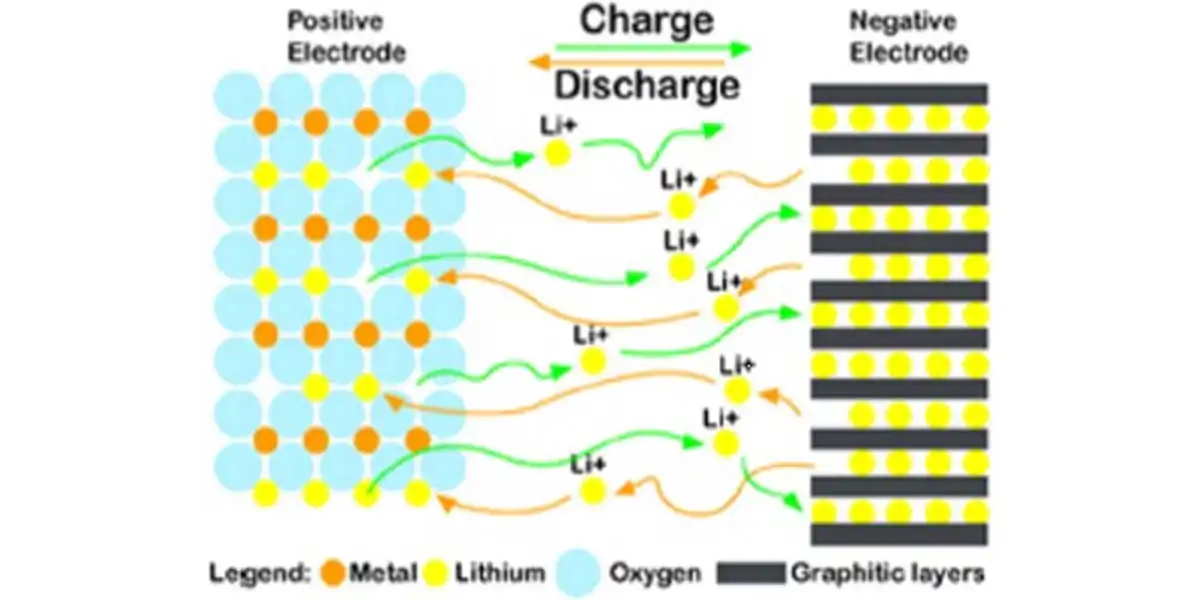
Lithium ion batteries (Li-ion) components include: a carbon (graphite) negative electrode, a metal-oxide positive electrode, an organic electrolyte (ether) with dissolved lithium ions and a microporous polymer separator. When the battery is charging, lithium ions flow from the positive metal oxide electrode to the negative graphite electrode. When the battery is discharging the reverse flow of ions takes place, Maara
explains.
Compared to lead-acid batteries, Li-ion batteries are a much less mature technology, says Maara. Li-ion batteries are mostly used in consumer electronics because of their high energy density (low weight), low standby losses, and cycling tolerance. Interest in utility-scale Li-ion batteries did not begin until 1970s, but little new technological development occurred until recent years.
“Since 2000, Li-ion batteries have become a popular choice for electric vehicle and aerospace applications,” Maara observes. “This resurgence in research and development of Li-ion technology for electric vehicle purposes has also led to interest in demonstrating the battery’s potential to perform utility functions.”
LITHIUM-ION BATTERY: MARKET OUTLOOK
Lithium-ion batteries dominate the portable electronics market. They offer two to four times the number of charge/discharge cycles and store more power than lead-acid batteries,
according to Maara. But they cost about four to six times more than a lead-acid battery of the same capacity.
“The cost differential is not as apparent with small batteries for phones, computers, for examples, but for industrial storage purposes, the cost per kilowatt hour (kWh) stored increases much more than other chemistries,” he says. “Lithium-iron phosphate batteries are being examined as the next generation of lithium technology.”
As such, the use of lead-acid electrochemical batteries has been declining in recent years as other chemistries (lithium-ion and sodium-sulfur) become more popular and advances in these chemistries make them more competitive, Maara notes. He anticipates that lithium-ion will become the preferred grid-scale battery storage technology by 2025.
The lithium-ion battery market size reached $257 million in 2015. Maara
forecasts the market will grow at a compound annual growth rate of 21.9% over the next decade to reach nearly $1.9 billion in 2025, chiefly because the batteries have reached grid-scale commercial viability for certain applications, with several manufacturers moving beyond merely selling batteries to offering integrated storage-related services, he maintains.