
Nov 8, 2016
Blog Life Sciences , Healthcare Companion Diagnostics for Antidepressants to Potentially Lift Patients
The rising prevalence of chronic diseases, an increase in disability-adjusted life years, and advancements in medical device technology are among key growth factors of the global market for emerging medical device technologies. According to a new study by BCC Research, high-growth segments include electrophysiology, bioresorbable vascular scaffolds, transcatheter heart valve therapy and drug-coated balloons, point-of-care (POC) diagnostics, companion diagnostics, and advanced cancer molecular diagnostics.
“Most of the high-growth segments are expected to increase at a double-digit growth rate in the next five years,” says BCC Research analyst Rita Thakur. “The increasing burden of conditions such as cardiovascular disease, diabetes, and cancer, as well as the adoption of advanced medical technologies, are key drivers.” BCC Research anticipates the global market for emerging medical device technologies to grow from $55.9 billion in 2016 to $90.8 billion by 2021, reflecting a five-year compound annual growth rate (CAGR) of 10.2%.
Additional drivers include an increasing global population, rising healthcare needs, the increased healthcare-spending capability of consumers, an improving healthcare infrastructure, and expanding investments by leading players and respective government agencies in the United States and Europe. Meanwhile, emerging economies are also receiving increasing investments from government bodies and private players in healthcare sectors, which is creating growth opportunities. In the United States, uncertainty concerning reimbursement and excise tax on medical devices are constraints.
Consumer adoption of more sophisticated devices to self-monitor health conditions is resulting in a higher demand for self-medication and home-based treatment (see Home Users Are Catalyst for Patient Monitoring Device Market). Also, remote communication technology allows healthcare professionals to support home-based patients (see Pharma Thinks Beyond the Pill: Connected Devices are a Smart Strategy). These technological changes are expanding the market because they offer convenient and efficient diagnosis or treatment at reduced healthcare costs.
As a segment, professional POC technology should reach $14.1 billion by 2021, up from $8.5 billion in 2016, demonstrating a five-year CAGR of 10.5%. The companion diagnostics technology segment is predicted to achieve $2.4 billion and $6.6 billion in 2016 and 2021, respectively, growing at a five-year CAGR of 22%. Oncology, inflammation, and autoimmune diseases are expected to lead the way in the growth of companion diagnostics. Other areas of projected interest are companion diagnostics for anticoagulants, antipsychotics, and antidepressants.
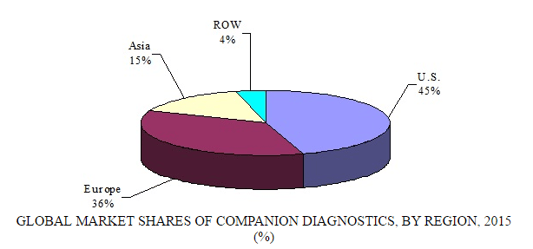
GLOBAL MARKET SHARES OF COMPANION DIAGNOSTICS BY REGION, 2015
COMPANION DIAGNOSTICS TECHNOLOGY FOR ANTIDEPRESSANTS
Depression is a major public health problem, affecting 17 million people in the United States every year. Less than one-third of people with the disorder find relief with the first antidepressant that is prescribed for them. Furthermore, patients must wait weeks to months to find out if the antidepressant will work for them. Two new studies, both focused on specific activities in the brain, offer hope for being able to more quickly match patients with an antidepressant that will be effective.
ORDER AN ELECTROENCEPHALOGRAM
UCLA researchers reported that a simple biomarker—a pair of brain-wave recordings (or electroencephalograms)—can show whether an individual is responding favorably to an antidepressant after just one week of treatment. An electroencephalogram can be performed in a doctor’s office and takes approximately 10 minutes. “Knowing whether a medication is going to work could eliminate weeks of waiting by the patient, and get them on effective treatment more quickly,” said Dr. Andrew Leuchter, senior author of the study and a professor in the Department of Psychiatry and Biobehavioral Science at UCLA’s Semel Institute.
The researchers used the electroencephalogram recordings to predict recovery from depression in patients taking escitalopram (Lexapro), which increases levels of serotonin in the brain. Serotonin levels in the brain maintain the ratio between slow brain waves (so-called delta–theta activity) and faster “alpha” brain waves. The brain uses this ratio between fast and slow waves to form chemical or electrical networks that support normal mood and thinking. Leuchter and his colleagues reasoned that the effect of escitalopram in shifting the balance between delta–theta and alpha activity would predict the effectiveness of the drug in relieving symptoms of depression.
The researchers tested whether brain-wave recordings in the first week of treatment would show that the antidepressant (compared with a placebo) corrected the frequency imbalance and predict a beneficial effect of the medication on an individual’s depression after seven weeks of treatment. Researchers analyzed data from 194 people with major depressive disorder. All patients were given an electroencephalogram before being given either escitalopram or placebo, and a second one after either one week of treatment or one week after taking the placebo.
After one week of taking escitalopram, the brain-wave recordings of the people who eventually responded favorably to the treatment differed significantly from the measurements of those who did not benefit. Specifically, those subjects who showed a large shift toward producing more delta–theta waves were less likely to enter remission with escitalopram treatment. Conversely, those subjects who shifted toward producing more alpha oscillations after one week of treatment with escitalopram were significantly more likely to find relief from their depression. Brain-wave shifts did not predict treatment outcomes in patients given a placebo.
Currently, finding the right antidepressant for individuals is a process of prolonged trial and error, Leuchter said. “As a result, it can take well over a year to recover from depression. Our biomarker could greatly shorten the length of time—to as little as one week—that a patient has to wait to see if a drug is going to work.” The study was published in the Journal of Psychiatric Research. The researchers plan to use brain-wave recordings to evaluate other antidepressant medications. [Full story is available at UCLA Newsroom].
OR, ORDER A BRAIN SCAN
By analyzing patients’ brain functions and personal histories, researchers at Stanford University were able to predict with 80% accuracy whether antidepressants would help them. “Currently, finding the right antidepressant treatment is a trial-and-error process because we don’t have precise tests,” said Leanne Williams, PhD, professor of psychiatry and behavioral sciences and the study’s senior author. “For some people, this process can take years. As a result, depression is now the leading cause of disability.”
The researchers conducted brain scans in 80 participants with depression. While undergoing functional MRI, patients viewed images of happy and fearful faces. Each face triggered brain circuits involving the amygdala, which is linked to the experiencing of emotions. The scans were conducted before and after an eight-week treatment period with three commonly used antidepressants: sertraline (Zoloft), escitalopram (Lexapro), and venlafaxine (Effexor). Participants also completed a 19-item questionnaire on early life stress. The researchers analyzed the pretreatment imaging and the questionnaire to predict how individual patients would respond immediately after the eighth week of treatment.
Study results showed that participants exposed to a high level of childhood trauma were most likely to recover with antidepressants if the amygdala was reactive to the happy faces. Those with a high level of childhood trauma whose amygdala showed low reactivity to the happy faces were less likely to recover with antidepressants. “We were able to show how we can use an understanding of the whole person—their experiences, their brain function, and the interaction between the two—to help tailor treatment choices,” Williams said.
The researchers say results from this study (published October 10 in the Proceedings of the National Academies of Science) could be useful for physicians who usually provide the first line of treatment for patients with depression. They envision the integrated clinic of the future in which physicians ask about childhood trauma and order a five-minute brain scan to help determine the best line of treatment. “If we are thinking about trying to get this right the first time, it’s useful to consider the option of ordering a scan,” Williams said.
Today, if the first line of treatment doesn’t work, patients spend an average of two to three years going through a trial-and-error period before getting treatment that helps, Williams said. She added that by that time, the disability burden has increased tremendously, with lost productivity of up to $14,000 per year per employee, in addition to the fact that the patient’s suffering continues while the disease progresses. [Full story is available at Stanford Medicine News Center.]
Pain management is a critical aspect of healthcare, touching the lives of millio...

What is interoperability in healthcare? Interoperability in healthcare is a crit...
In recent years, the integration of Microelectromechanical Systems (MEMS) device...

We are your trusted research partner, providing actionable insights and custom consulting across life sciences, advanced materials, and technology. Allow BCC Research to nurture your smartest business decisions today, tomorrow, and beyond.
Contact UsBCC Research provides objective, unbiased measurement and assessment of market opportunities with detailed market research reports. Our experienced industry analysts assess growth opportunities, market sizing, technologies, applications, supply chains and companies with the singular goal of helping you make informed business decisions, free of noise and hype.